650
Views & Citations10
Likes & Shares
Salt stress is one of the most important environmental factors that negatively influence plant’s different processes and reduce plant growth. Exogenous application of polyamines to stress treated plants could lead to alleviate stress injury in plants and helps them to grow and yield better. Present experiment was carried out to assess the protective role of spermidine on Helianthus annuus grown under salinity stress. Plants were irrigated with NaCl concentrations (50 mM, 100 mM and 150 mM) and three different doses of spermidine (0.1 mM, 1 mM and 2 mM) were foliarly applied on plants. Salt stress caused significant reduction in growth parameters (Plant height, root length, number of leaves, fresh and dry biomass, seed number and weight per plant), oil quantity, endogenous IAA content, content of chlorophyll, reducing and non-reducing sugars, total carbohydrates and total proteins. Results indicated promotion in secondary metabolites (phenols, flavonoids, lycopene, beta carotene, terpenoids and total antioxidants) in plants under same salt stress doses. Spermidine application induced stimulatory effect and improved above mentioned parameters before and after salt treatment. So, results for physiological and metabolic analysis of sunflower in present investigation elucidate spermidine foliar application as salt tolerance strategy under saline environment.
Keywords: Spermidine, Salinity, Sunflower, Phenols, Antioxidants, Terpenoids
INTRODUCTION
Abiotic stress results not only crop yield reduction but also damage the plants and these are the basic symptoms of different abiotic stresses [1]. Different abiotic stresses are the major threats towards the living world and specially the plant kingdom. Plants have developed different physiological, biochemical and metabolic strategies in order to tolerate these abiotic stresses. Activation and deactivation of the complex signaling pathway in response to different abiotic stresses is difficult to predict [2]. Worldwide potential threat to different soil is salinity which results in the reduction of crops yield than expected level. This stress causes crop growth reduction and limited yield in different ways. Two basic and primary effects of salinity on plants are ionic toxicity and osmotic stress that consequently results in some alternate effects like reduction in expansion of cell, proper function of membrane and production of assimilates along with some minor observations like decrease in cytosolic metabolism and ROS production.
Group of natural compounds with structures containing aliphatic nitrogen are known as polyamines. Polyamines are present almost in all living-organisms and play crucial roles in different physiological processes like response to environmental stresses and cell development and growth. Form of polyamine that are commonly found in higher plants are Putricine, Spermidine and Spermine and they are found in insoluble bound form, soluble conjugated form and free form [3]. Soluble conjugated form of polyamines are covalently conjugated to small molecules such as phenolic compounds, while insoluble bound PAs are covalently bound to macromolecules such as nucleic acids and proteins [4]. There are also uncommon form of polyamines present in living system, like 1,3-diaminopropane,homospermidine, canavalmine and cadaverine have been detected in many biological systems, including bacteria, algae, animal and plants. Polyamines are found as cations at the physiological pH.
The sunflower (Helianthus annuus L.) being a member of Asteraceae provides a vital opportunity to assess such trade-offs between growth and resistance towards stress. This is due to presence of different forms of the same species exist as domesticated crop, agricultural weed as well as a wild species in natural areas. If we compare between wild and domesticated sunflower, it was observed that wild sunflower produces branched and short stems and many small flowering heads while on the other hand, a domesticated sunflower develops a tall un-branched stem with a single and very large head. Interestingly, a third and weedy form of sunflower arises as a pest in croplands and highly disturbed areas and is found throughout North America [5,6], Argentina [7] and in much of Europe [8-10]. Sunflower weeds found in agricultural fields possess morphology generally intermediate between wild and domesticated varieties with tall but branched stems [11]. In the present study, the plant Helianthus annuus was selected based on its wide range of enthnobotanical importance, to determine the role of spermidine concentration as foliar spray in growth and biochemical attributes while plant grown under salt stress condition.
MATERIALS AND METHODS
Present study was designed to investigate the effect of polyamine on growth attributes, chlorophyll a, chlorophyll b, chlorophyll a/b ratio, total chlorophyll, total carotenoids, reducing sugar, non-reducing sugar, total carbohydrates, total proteins, total phenols and total antioxidants of Helianthus annuus under salinity stress. Seeds of Helianthus annuus were obtained from the local market of Mardan, Khyber Pukhtunkhwa. This experiment was designed in completely randomized manner and comprised of 48 pots which were further divided into 4 sets. Details of 4 sets were as follows:
1st set: Without polyamine (spermidine) comprising of control and three salinity treatments (50 mM, 100 mM and 150 mM).
2nd set: Polyamine provided as spermidine 0.1 mM the set comprises of control and three salinity treatments (50 mM, 100 mM and 150 mM).
3rd set: Polyamine provided as spermidine 1 mM the set comprises of control and three salinity treatments (50 mM, 100 mM and 150 mM).
4th set: Polyamine provided as spermidine 2 mM the set comprises of control and three salinity treatments (50 mM, 100 mM and 150 mM).
45 cm deep plastic pots were used in this experiment. Out of 48 pots, 12 pots were placed in each set and 3 replicates were maintained for each treatment i) control (non-saline); ii) 50 Mm NaCl; iii) 100 mM NaCl and iv) 150 mM NaCl. Every pot was filled with washed sandy loam soil. Soil in each pot was saturated with full strength Hoagland’s solution. Approximately, uniform size and equal number of seeds were surface sterilized with 0.1% mercuric chloride for one minute and then washed with distilled water. 4 seeds were sown in each pot. They were irrigated with an equal amount, i.e., 50 ml of tap water on daily basis. Accordingly, when seedlings reached at 3 leaves stage, they were thinned out as one seedling per pot. At the end, 48 pots were then arranged in a completely randomized design (CRD) in the Botanical Garden of Department of Botany, Abdul Wali Khan University, Mardan and salt treatment was given and then each pot was irrigated with 1.5 L of tap water twice a week. On completion of salt treatment in pots, different concentrations of polyamine (spermidine) were applied foliarly in different sets.
Agronomic traits
Agronomic parameters were measured at termination of experiment and plant height, root length, number of leaves, fresh and dry biomass, number of seed/plant and seed weight/plant were recorded.
Biochemical analysis
Leaves sample were collected at a grand period of growth for the estimation of chlorophyll a, chlorophyll b, total chlorophyll, reducing sugars, non-reducing sugars, carbohydrates, proteins, phenols and total antioxidant status.
Extraction and estimation of chlorophyll
Estimation of chlorophyll was performed by a spectrophotometric method described by Lichtenthaler [12].
Determination of reducing and non-reducing sugars
Determination of reducing sugars was performed by spectrophotometric method described by Miller [13].
Extraction and estimation of total carbohydrates
The amount of total carbohydrates was analyzed using the spectrophotometric method of Dey [14].
Determination of proteins
The method is rapid and sensitive for the quantification of microgram quantities of proteins utilizing the principle of protein-dye binding [15].
Endogenous IAA determination
For determination of indole acetic acid (IAA) plant samples were analyzed by (high performance liquid chromatography) HPLC, as described by Rivier and Crozier [16].
Total phenol determination
The amount of total phenolics was analyzed using the Folin-Ciocalteu (FC) spectrophotometric method described previously by Elzaawely and Tawata [17].
Lycopene and β carotenes
β-carotene and lycopene were determined according to the method of Karnjanawipagul et al. [18].
Total terpenoids
Total terpenoids in plants were determined according to the method of Ghorai et al. [19].
Total antioxidants
The ferric ion reducing power capability of samples was determined by using modified spectrophotometric method of Yen and Chen [20].
Experimental design and statistical analysis
The experimental design was Completely Randomized Design (CRD) bearing three salt levels replicated thrice. Results were analyzed by one-way ANOVA using SPSS 21.0 statistical software and significant differences between the means of parameters were determined by using the Duncan’s Multiple Range Test (P<0.05).
RESULTS AND DISCUSSION
Growth analysis
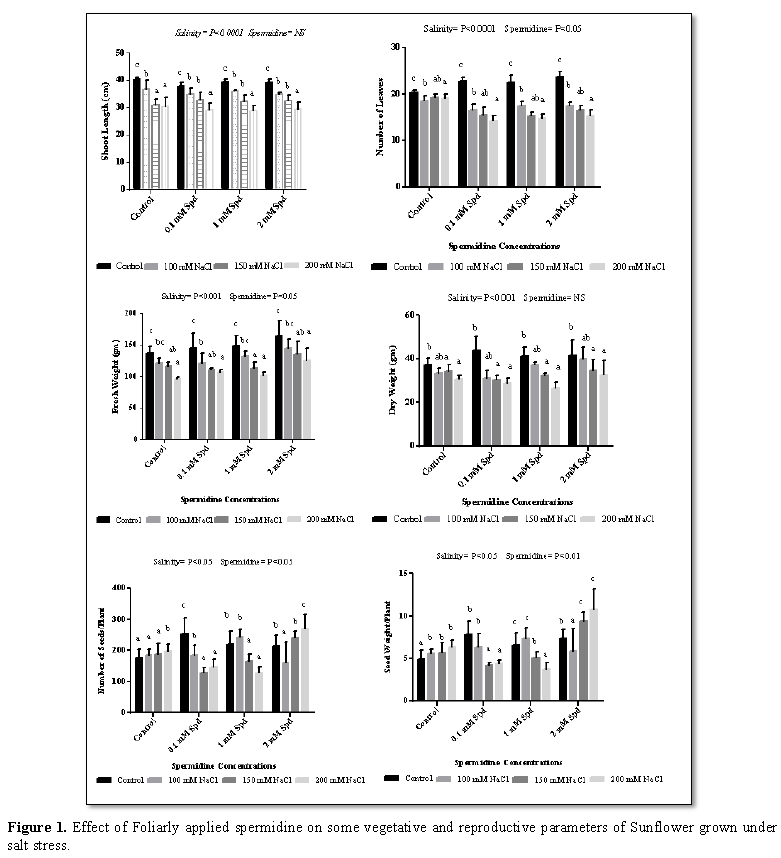
Salinity reduces plant growth through osmotic and toxic effects and high sodium uptake ratio values cause sodicity, which increases soil resistance, reduces root growth and reduces water movement through the root with a decrease in hydraulic conductivity [21]. In present investigation shoot length, number of leaves, fresh weight and dry weight were reduced significantly with salinity (100 mM, 150 mM and 200 mM) in all sets as compare to control (Figure 1). Seed number and weight per plant was decreased in all sets except control which showed increase with salinity as compared to control. Growth retardation could be attributed to the inhibition of cell elongation [22]. Stress induced reduction in protein synthesis may affect plant growth. Accumulation of amino acids reduces the osmotic potential which facilitates the inward movement of the water [23,24]. The inhibitory effect due to saline environment was probably due to the impact of salt on the stomata and photosynthesis process, as intercellular CO2 concentration was reduced and photosynthetic enzymes, chlorophylls and carotenoids were disturbed, respectively [25]. Plant growth is limited by a reduction in the photosynthesis rate and by an excessive uptake of salts affects the production of specific metabolites that directly inhibit growth [26]. Different concentration of spermidine (0.1 mM, 1 mM and 2 mM) significantly reduced shoot length and fresh weight under saline (100 mM and 200 mM) and non-saline conditions except 150 mM NaCl which showed increase as compare to control (Figure 1). Application of different concentration of spermidine (0.1 mM, 1 mM and 2 mM) significantly increased number of leaves, dry weight and seed weight under non-saline and 100 mM salt while it showed significant decrease at 150 and 200 mM salt. Different concentration of spermidine (0.1 mM, 1 mM and 2 mM) significantly increased number of seeds under non saline and saline condition except 20 mM which showed reduction. Spermidine application in salinized nutrient solution during short-time stress resulted in alleviation of the salinity induced membrane damage in the roots and plant growth and photosynthesis inhibition, together with an increase in polyamine and proline contents and antioxidant enzyme activities in the roots [27].
Photosynthetic pigments
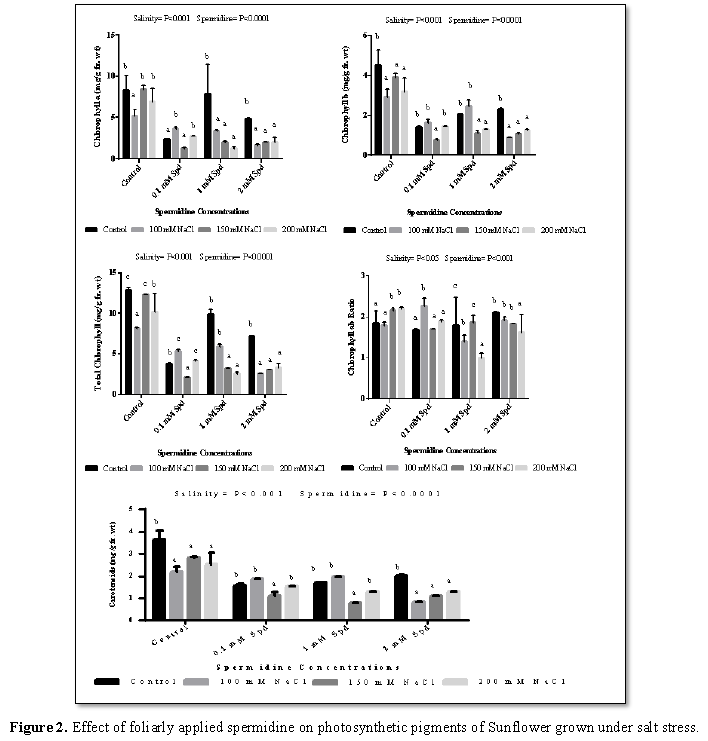
Chlorophyll a, chlorophyll b and total chlorophyll reduced significantly with salinity (100 mM, 150 mM and 200 mM) in all sets as compare to control except 0.1 mM spermidine which showed increase in chlorophyll b and total chlorophyll with salinity (Figure 2). The decrease in chlorophyll levels in salt stressed plants has been considered as a typical symptom of oxidative stress [28] and was attributed to the inhibition of chlorophyll synthesis, together with the activation of its degradation by the enzyme chlorophyllase [29]. Reduction of chlorophyll contents, as a result of either slow synthesis or fast breakdown, indicated that there was a photoprotection mechanism through reducing light absorbance by decreasing chlorophyll contents [30]. Reudction in photosynthetic pigments and carotenoids was confirmed by the study of Almodares et al. [31] on sorghum and Sofy [32] on lentil. Different concentration of spermidine (0.1 mM, 1 mM and 2 mM) significantly reduced chlorophyll a, chlorophyll b and total chlorophyll under non-saline and saline condition as compared to control. a/b ratio was reduced after application of different concentration of spermidine under saline and non-saline condition except 100 mM salt which showed increase (Figure 2). Gupta et al. [33] observed in wheat plants that polyamines application increases the total chlorophyll contents. According to Besford et al. [34] different molecular complexes that are present in thylakoid membrane stabilized after application of putrescine. Duan et al. [27] suggested foliar application of different polyamines on plants which results in alleviation in salt induced reduction up to certain extent in photosynthetic efficiency. They further commented that this phenomenon strongly depends on polyamine type, concentration and salt stress level. Putrescine capability (as polyamine) to stabiles protoplast and prevent both loss of chlorophyll during senescence in protoplast and leaves [35]. Ma et al. [36] suggested that the effect of polyamines in inhibiting chlorophyll degradation may be related to the inhibition of peroxidase activity. Increasing carotenoids content may due to converting these substances to pyruvic acid that led to enhance biosynthesis of leaf carotenoids [37].
Reducing sugars and non-reducing sugars
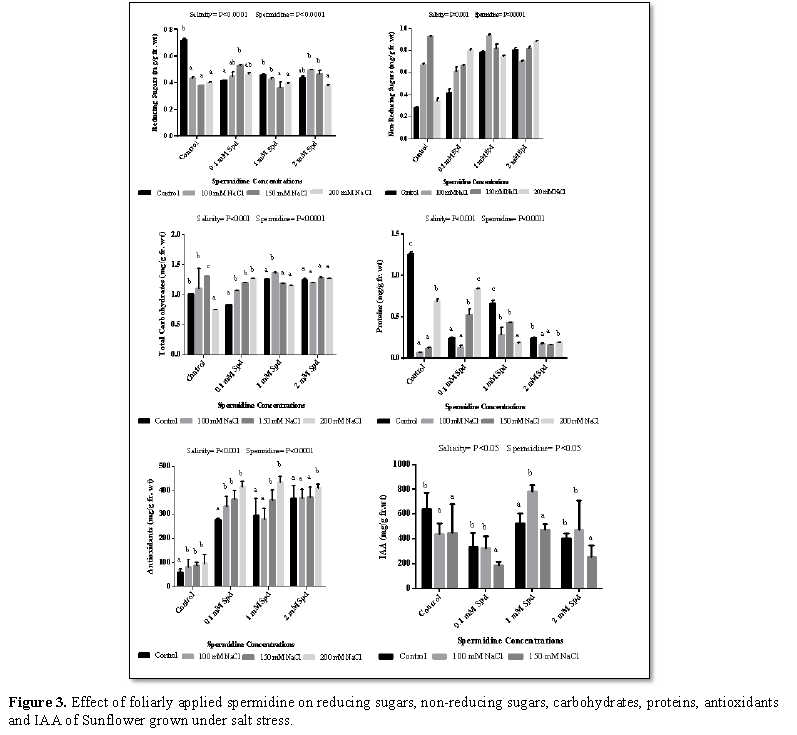
Reducing sugars were reduced significantly with salinity (100 mM, 150 mM and 200 mM) in control and 1 mM spermidine while it shows increase in 0.1 mM and 2 mM sets as compare to control. Non-reducing sugars increased significantly with salinity (100 mM, 150 mM and 200 mM) in all sets as compare to control (Figure 3). In plants, under salinity stress conditions, accumulation of sugars (reducing, non-reducing) is reported which allowed the plants to adjust osmotically [38,39]. On the other hand, Mostafa [40] observed that at low and moderate salinity levels, sugars and consequently the total carbohydrates are decreased. Different concentration of spermidine (0.1 mM, 1 mM and 2 mM) significantly reduced reducing sugars under control and 200 mM salt condition while it shows increase less than 100 mM and 150 mM salt stress as compare to control. Application of different concentration of spermidine increased non-reducing sugars under saline and non-saline conditions (Figure 3). Liu et al. [41] found that spraying Brassica plants with PAs gave the highest value of total soluble sugar percentage. PAs alleviated the adverse effects of salt stress on total soluble sugars concentration and this is at least partially due to enhanced amylase activity and chlorophyll content, reported as a response [42]. Hussein et al. [43] reported that increasing the sugars concentration by putrescine application may be due to the role of putrescine on improving plant growth; carbohydrate content resulted in increasing plant tolerance to salinity stress.
Carbohydrates
Carbohydrates increased significantly with salinity (100 mM, 150 mM and 200 mM) in all sets except 1 mM spermidine set which show reduction as compare to control (Figure 3). Plants have been attributed an adaptation by increase in carbohydrate level in response to stresses [23]. High carbohydrate content under salt stress prevents plants from the oxidative damage and maintains structure of proteins and membranes [44]. Increase in carbohydrate content depends on environmental conditions, species and genotypes within the same species [45]. Application of different concentration of spermidine increased total carbohydrates under non saline and saline condition except 150 mM salt which showed reduction as compare to control (Figure 3). In this regard, several investigators proved that polyamine plays an important role in carbohydrate metabolism. El-Bassiouny et al. [46] found that application of PAs treatment had favorable effect on the synthesis and accumulation of carbohydrates in leaves of wheat plants. In most cases, plants sprayed with PAs at the different concentrations had higher contents of total carbohydrates in their leaves, compared to the untreated control.
Proteins
Total proteins reduced significantly with salinity (100 mM, 150 mM and 200 mM) in all sets except 0.1 mM spermidine set which showed increase as compare to control (Figure 3). Stressed plants mostly exhibited nutrient imbalance which causes inhibition in protein synthesis delay in enzyme solubilization and reduction in enzymatic activities [47]. This decrease was may be due to the increasing activity of acid and alkaline proteases in order to keep osmotic stress during NaCl stress [48]. Our results are also in agreement with Kennedy and De Fillippis [49] who studied Grevillea ilicifolia and G. arenaria under saline stress. Application of different concentration of spermidine reduced total proteins under non saline and 200 mm saline condition while under 100 mM and150 mM salt total proteins showed increase as compare to control (Figure 3). These results are in harmony with those obtained by Amira Abdul Qados [50] on mung bean, where they found that, contents of proteins increased as a result of PAs application with saline soil. El-Bassiouny et al. [46] indicated that PAs was the most effective compound in increasing soluble carbohydrate, poly saccharides, total carbohydrates, proline, total amino acid and protein contents of wheat plants and grains under normal or stressed condition. Exogenous polyamines stimulated protein synthesis. In addition, increased polyamine biosynthesis is correlated with enhanced protein and nucleic acid synthesis during cellular proliferation in a variety of prokaryotic and eukaryotic organisms. Polyamines have long been known to stimulate protein synthesis when added to cell-free translation systems.
IAA
IAA reduced significantly with salinity (100 mM, 150 mM) in control and 0.1 mM set while 1 mM and 2 mM set showed increase at 100 mM and decrease at 150 mM salt as compared to control (Figure 3). Abiotic stresses, like salinity can influence IAA homeostasis due to alterations in IAA metabolism and distribution. Moreover, generation of ROS in response to abiotic stresses may also influence auxin response. Variations in indole-3-acetic acid (IAA) level in Funalia trogii under salt stress. Application of different concentration of spermidine significantly increased IAA under non saline and 150 mM saline condition while 100 mM salt showed significant reduction as compare to control. The polyamines have the potential of conjugates formation with phenols; thereby increase IAA biosynthesis. Previous results also demonstrated the increase in IAA contents of putrescine treated Chicory (Cichrium intybus L.) [51]. Increase in IAA and GA contents is also in agreement with Nassar et al. [52] and El-Bassiouny [53]. The increase in the plant growth regulators is correlated positively with plant height, fresh weight and could be attributed to increase cell division and cell proliferation [46].
Total phenols
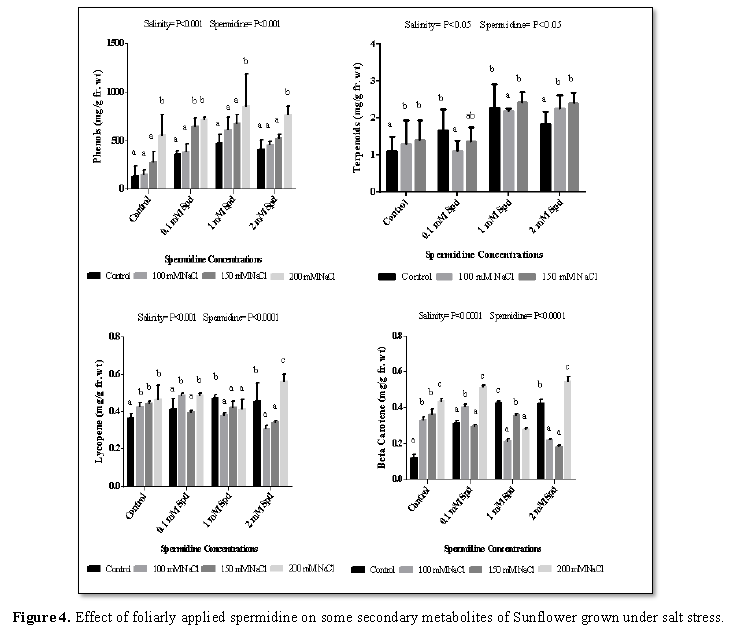
Phenols act as free radical scavengers as well as substrates for many antioxidant enzymes [54]. Total phenols increased significantly with salinity (100 mM, 150 mM and 200 mM) in all sets as compared to control (Figure 4). It is well established that abiotic stresses including salinity cause oxidative damages, mainly by generating excess ROS (reactive oxygen species), which can attack lipids, proteins, DNA and carbohydrates. The ROS is comprised of both non-radical (molecular) (1O2 and H2O2) and free radical forms (OH, O2−, RO and HO2•) [55]. In order to scavenge or detoxify ROS, antioxidants such as phenolic compounds are produced by plants [56] and this is why the biosynthesis of such compounds is generally stimulated in salt-exposed plants [57]. In previous reports, the volume of phenolic compounds increased in buckwheat sprout [58], Salvia mirzayanii [59] and Carthamus tinctorius flowers [60] under NaCl stress. Application of different concentration of spermidine increased total phenols under non saline and saline condition as compared to control. Agastian et al. [61] deduced that application of various concentrations of PAs induced high significant increases in the total phenol content of cowpea shoots. This increase in total phenol contents in response to PAs treatments concurrently with increase in IAA contents in shoots of cowpea plants led to the suggestion that most of phenol compounds are diphenols and polyphenols which may inhibit IAA oxidase activity resulting in auxin accumulation, which reflected in stimulated growth and yield of the treated plants. Moreover, Neveen [62] observed that putrescine foliar application 1 or 2 ppm enhanced total soluble phenols in sweet pepper shoots.
Lycopene
Lycopene is a highly important bioactive compound that has the capacity to combat oxidative stress [63]. Lycopene and β-carotene are widely known as powerful natural antioxidants that act as the most efficient singlet oxygen quenchers in vitro among common carotenoids [64]. Lycopene increased significantly with salinity (100 mM, 150 mM and 200 mM) in all sets except 2 mM set which shows reduction as compared to control (Figure 4). Increase in lycopene content following salt stress has been reported [65], which is in agreement with the present study. Application of different concentration of spermidine increase lycopene under non-saline and 200 mM salt stress while it shows reduction under 100 mM and 150 mM salt stress conditions. Lycopene is an antioxidant carotenoid pigment that protects against cell damage. Løvaas [66] investigated the anti-oxidative effects of spermine, spermidine and putrescine by measurement of primary and secondary oxidation products of polyunsaturated fatty acids. It was demonstrated that polyamines inhibit the oxidation of polyunsaturated fatty acids, a-tocopherol and carotenoid pigments. Increase in the lycopene could be due to inhibition of its oxidation after foliar application of spermidine.
Beta carotene
Lycopene is a highly important bioactive compound that has the capacity to combat oxidative stress [63]. Lycopene and β-carotene are widely known as powerful natural antioxidants that act as the most efficient singlet oxygen quenchers in vitro among common carotenoids [64]. Beta carotene increased significantly with salinity (100 mM, 150 mM and 200 mM) in control and 0.1 mM spermidine set while 1 mM and 2 mM spermidine set showed significant reduction as compared to control (Figure 4). Beta-carotene is known as a strong antioxidant and is the best quencher for singlet oxygen [63]. Application of different concentration of spermidine increase beta carotene under non-saline and 200 mM salt stress while it shows reduction under 100 mM and 150 mM salt stress conditions. Put application increased the activity of antioxidant enzymes and carotenoids in leaf tissues of salt stressed Brassica juncea seedlings and enhanced seedling growth relative to the untreated controls [67]. Together, these studies indicate that altering polyamine accumulation through manipulation of biosynthetic pathways or direct application could have an effect on physiological responses to salt stress. Exogenously applied polyamines alleviate salt resistance via the modulation of cellular anti-oxidative components (enzymatic or non-enzymatic) [68].
Total terpenoids
Total terpenoids increased significantly with salinity (100 mM, 150 mM and 200 mM) in all sets except 0.1 mM set which shows reduction as compared to control (Figure 4). Plant SMs are usually classified according to their chemical structure [69]. Several groups of large molecules, including phenolic acids and flavonoids, terpenoids, steroids and alkaloids have been implicated in activation and reinforcement of defense mechanisms in plants [69,70]. For most plants, external factors or variables (light, temperature, soil water, soil fertility and salinity) can significantly affect some processes associated with growth and development of the plants, even their ability to synthesize secondary metabolites, eventually leading to the change of overall phytochemical profiles which play a strategic role in production of bioactive substances [71-73]. In other words, plant secondary metabolites can be gradually generated in response to environmental stress and hence plant secondary metabolism be viewed as plant behavior that is in part the ability of adaptation and survival in response to environment stimuli during the lifetime [74] and serve to establish ecological relationships between plants and other organisms [75]. Application of different concentration of spermidine increase total terpernoids under saline and non-saline conditions (Figure 4). Accumulation of metabolites often occurs in plants subjected to stresses including various elicitors or signal molecules [76].
Total antioxidant
Total antioxidant increased significantly with salinity (100 mM, 150 mM and 200 mM) in all sets as compared to control (Figure 3). The degree of cellular oxidative damage in plants exposed to abiotic stress is controlled by the capacity of the plants to produce antioxidant agents. Therefore, salt tolerance seems to be favored by the increase in plant antioxidant levels to detoxify the reactive oxygen species produced under these conditions. Application of different concentration of spermidine increased total antioxidant under different concentration of salt while under non saline condition total antioxidant was reduced after application of different concentration of spermidine (Figure 3). PAs are known to significantly enhance activity of both enzymatic [77] and non-enzymatic [78] antioxidants. Therefore, the PA control over the balance between ROS production and scavenging may “shape” H2O2 signal, conferring differential stress responses between species and genotypes. PAs may influence various antioxidant enzymes through regulation of their expression. Higher transcript levels of antioxidant enzyme-encoding genes have been detected in tissues treated with exogenous PAs or in the transgenic plants overexpressing PA biosynthetic genes [79,80].
1. Tardieu F, Tuberosa R (2010) Dissection and modeling of abiotic stress tolerance in plants. Curr Opin Plant Biol 13: 206-212.
2. Chawla K, Barah P, Kuiper M, Bones AM (2011) Systems biology: A promising tool to study abiotic stress responses. Omics and Plant Abiotic Stress Tolerance, pp: 163-172.
3. Lefevre I, Gratia E, Lutts S (2001) Discrimination between the ionic and osmotic components of salt stress in relation to free polyamine level in rice (Oryza sativa). Plant Sci 161: 943-952.
4. Duan J, Li J, Guo S, Kang Y (2008) Exogenous spermidine affects polyamine metabolism in salinity stressed Cucumis sativus roots and enhances short-term salinity tolerance. J Plant Physiol 165: 1620-1635.
5. Massinga RA, Al-Khatib K, Amand PS, Miller JF (2003) Gene flow from imidazolinone-resistant domesticated sunflower to wild relatives. Weed Sci 51: 854-862.
6. Kane NC, Rieseberg LH (2007) Selective sweeps reveal candidate genes for adaptation to drought and salt tolerance in common sunflower, Helianthus annuus. Genetics 175: 1823-1834.
7. Poverene M, Cantamutto M (2010) A comparative study of invasive Helianthus annuus populations in their natural habitats of Argentina and Spain. Helia 33: 63-74.
8. Faure N, Serieys H, Berville NA (2002) Potential gene flow from cultivated sunflower to volunteer, wild Helianthus species in Europe. Agric Ecosyst Environ 89: 183-190.
9. Holec J, Soukup J, Cerovska M, Novakova K (2005) Common sunflower (Helianthus annuus var. annuus) – Potential threat to coexistence of sunflower crops in Central Europe. In: Proceedings 2nd Internal Conference on co-existence between GM and non-GM based agricultural supply chains, Montpellier, France 14-15: 271-272.
10. Vischi M, Cagiotti ME, Cenci CA, Seiler GJ, Olivieri AM (2006) Dispersal of wild sunflower by seed and persistent basal stalks in some areas of Central Italy. Helia 29: 89-94.
11. Muller MH, Delieux F, Fernandez-Martinez JM, Garric B, Lecomte V, et al. (2009) Occurrence, distribution and distinctive morphological traits of weedy Helianthus annuus L. populations in Spain and France. Genet Resour Crop Evol 56: 869-877.
12. Lichtenthaler HK (1987) Chlorophylls and carotenoids: 30 pigments of photosynthetic biomembranes. Methods Enzymol 148: 350-382.
13. Miller GL (1972) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem 31: 426-428.
14. Dey PM (1990) Oligosaccharides. In: Methods in Plant Biochemistry: Carbohydrates. Vol. 2, Dey PM, editor. London: Academic Press, pp: 189-218.
15. Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
16. Rivier L, Crozier A (1987) Principles and practice of plant hormone analysis. Academic Press: London 2: 401.
17. Elzaawely AA, Tawata S (2012) Antioxidant capacity and phenolic content of Rumex dentatus L. grown in Egypt. J Crop Sci Biotechnol 15: 59-64.
18. Karnjanawipagul P, Nittayanuntawech W, Rojsanga P, Suntornsuk L (2010) Analysis of β-carotene in carrot by spectrophotometry. J Pharm Sci 37: 8-16.
19. Ghorai N, Chakraborty S, Gucchait S, Saha SK, Biswas S (2012) Estimation of total terpenoids concentration in plant tissues using a monoterpene, Linalool as standard reagent. Nature Protocol Exchange.
20. Yen GC, Chen HY (1995) Antioxidant activity of various tea extracts in relation to their anti-mutagenicity. J Agric Food Chem 43: 27-32.
21. Rengasamy P, Olsson KA (1993) Irrigation and sodicity. Soil Res 31: 821-837.
22. Bandeoglu E, Eyidogan F, Yucel M, Oktem HA (2004) Antioxidant responses of shoots and roots of lentil to NaCl-salinity stress. Plant Growth Regulation 42: 69-77.
23. Ashraf MY, Ashraf M, Sarwar G (2005) Response of Okra (Hibiscus esculentus) to drought and salinity stress. In: Vegetables: Growing Environment and Mineral Nutrition (Ed.): Dris R. WFL Publisher, Helsinki, Finland, pp: 166-177.
24. Balal RM, Ashraf MY, Khan MM, Jaskani MJ, Ashfaq M (2011) Influence of salt stress on growth and biochemical parameters of citrus rootstocks. Pak J Bot 43: 2135-2141.
25. Taârit MB, K Msaada, K Hosni & B Marzouk (2012) Physiological changes, phenolic content and antioxidant activity of Salvia officinalis L. grown under saline conditions. J Sci Food Agric 92: 1614-1619.
26. Mazher AA, El-Quesni EF, Farahat MM (2007) Responses of ornamental and woody trees to salinity. World J Agric Sci 3: 386-395.
27. Duan J, Li J, Guo S, Kang Y (2008) Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J Plant Physiol 165: 1620-1635.
28. Smirnoff N (1996) Botanical briefing: the function and metabolism of ascorbic acid in plants. Ann Bot 78: 661-669.
29. Santos CV (2004) Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Scientia Horticulturae 103: 93-99.
30. Elsheery NI, Cao KF (2008) Gas exchange, chlorophyll fluorescence and osmotic adjustment in two mango cultivars under drought stress. Acta Physiologiae Plantarum 30: 769-777.
31. Almodares A, Hadi MR, Dosti B (2008) Effect of salt stress on growth parameters and carbohydrates contents in sweet sorghum. Res J Environ Sci 2: 298-304.
32. Sofy MR (2011) Physiological and biochemical responses of plant to growth regulators and stress condition. PhD. Plant Physiology, Faculty of Science, Al-Azhar University.
33. Gupta NK, Gupta S, Shukla DS, Deshmukh PS (2003) Differential responses of BA injection on yield and specific grain growth in contrasting genotypes of wheat (Triticum aestivum L.). Plant Growth Regul 40: 201-205.
34. Besford RT, Richardson CM, Campos JL, Tiburcio AF (1993) Effect of polyamines on stabilization of molecular complexes in thylakoid membranes of osmotically stressed oat leaves. Planta. 189: 201-206.
35. Kaur-Sawhney R, Galston AW (1979) Interaction of polyamines and light on biochemical processes involved in leaf senescence. Plant Cell Environ 2: 189-193.
36. Ma JY, Zhou R, Cheng BS (1996) Effect of spermin on the peroxidase activity of detached wheat leaves. J Shandang Agric Univ 27:176-180.
37. Martin-Tanguy J (2001) Metabolism and function of polyamines in plants: Recent development (new approaches). Plant Growth Regul 34: 135-148.
38. Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell 14: 185-205.
39. Wang Z & GW Stute (2000) The role of carbohydrates in active osmotic adjustment in apple under water stress. J Am Soc Hort Sci 117: 816-823.
40. Mostafa DM (2004) Metabolic imbalance and salinity tolerance of two maize cultivars. M.Sc. Thesis. El-Minia Univ. Elminia, Egypt, pp: 1-195.
41. Liu AR, Zhang YB, Ling N (2002) Eeffect of spermine and spermidine on several physiological indexes of Brassica campestris. Plant Physiol Comm Chinese Acad Agric Sci (CAAS) Scientech Documentation and Information Center, Beijing, China, 38: 349-351.
42. Sood S, Nagar PK (2003) The effect of polyamines on leaf senescence in two diverse rose species. Plant Growth Regul 39: 155-160.
43. Hussein MM, El-Gfereadly NHM, El-Desuki M (2006) Role of putrescine in resistance to salinity of pea plants (Piscum sativum L.). J Appl Sci Res 2: 598-604.
44. Hajihashemi S, Kiarostami K, Enteshari S, Saboora A (2006) The effects of salt stress and paclobutrazol on some physiological parameters of two salt tolerant and salt sensitive cultivars of wheat. Pak J Biol Sci 9: 1370-1374.
45. Kameli A, Losel DM (1993) Carbohydrates and water status in wheat plants under water stress. New Phytol 125: 609-614.
46. El-Bassiouny HMS, Mostafa HA, El-Khawas SA, Hassanein RA, Khalil SI, et al. (2008) Physiological responses of wheat plant to foliar treatments with arginine or putrescine. Austr J Basic Appl Sci 2: 1390-1403.
47. Javed S, Bukhari SA, Ashraf MY, Mahmood S, Iftikhar T (2014) Effect of salinity on growth, biochemical parameters and fatty acid composition in safflower (Carthamus tinctorius L.). Pak J Bot 46: 1153-1158.
48. Parida A, Das AB, Das P (2002) NaCl causes changes in photosynthetic pigments, proteins and other metabolic components in the leaves of a true mangrove, Bruguiera parviflora, in hydroponic cultures. J Plant Biol 45: 28-36.
49. Kennedy BF, De Fillippis LF (1999) Physiological and oxidative response to NaCl of the salt tolerant Grevillea ilicifolia and the salt sensitive Grevillea arenaria. J Plant Physiol 155: 746-754.
50. Amira Abdul Qados MS (2010) Effect of arginine on growth, nutrient composition, yield and nutritional value of mung bean plants grown under salinity stress. Nat Sci 8.
51. Bais HP, Ravishankar GA (2002) Role of polyamines in the ontogeny of plants and their biotechnological applications. Plant Cell Tissue Organ Cult 69: 1-34.
52. Nassar AH, El-Tarabily KA, Sivasithamparam K (2003) Growth promotion of bean (Phaseolus vulgaris L.) by a polyamine producing isolate of Streptomyces griseoluteus. Plant Growth Regul 40: 97-106.
53. El-Bassiouny HMS (2004) Increasing thermotolerance of Pisum sativum L. plants through application of putrescine and stigmasterol. Egypt J Biotechnol 18: 93-118.
54. Martin-Tanguy J (2001) Metabolism and function of polyamine in plants. Plant Growth Regul 34: 135-148.
55. Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48: 909-930.
56. Petridis A, Therios I, Samouris G, Tananaki C (2012) Salinity-induced changes in phenolic compounds in leaves and roots of four olive cultivars (Olea europaea L.) and their relationship to antioxidant activity. Environ Exp Bot 79: 37-43.
57. Navarro JM, Flores P, Garrido C, Martinez V (2006) Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chem 96: 66-73.
58. Lim JH, Park KJ, Kim BK, Jeong JW, Kim HJ (2012) Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem 135: 1065-1070.
59. Valifard M, Mohsenzadeh S, Kholdebarin B, Rowshan V (2014) Effects of salt stress on volatile compounds, total phenolic content and antioxidant activities of Salvia mirzayanii. S Afr J Bot 93: 92-97.
60. Salem N, Msaada K, Dhifi W, Limam F, Marzouk B (2014) Effect of salinity on plant growth and biological activities of Carthamus tinctorius L. extracts at two flowering stages. Acta Physiol Plant 36: 433-445.
61. Agastien P, Kingsley SJ, Vivekanondon M (2000) Effect of salinity on photosynthesis and biochemical characteristics in mulberry genotypes. Photosynthetica, 38: 287-290.
62. Neveen BT (2003) Physiological studies on the effect of salinity, ascorbic acid and putrescine on sweet pepper plants. Ph.D. Thesis, Faculty of Agriculture, Cairo University, Egypt.
63. Raiola A, Rigano MM, Frusciante L, Barone A (2014) Enhancing the health promoting effects of tomato fruit for biofortified food. Mediators Inflamm, pp: 1-16.
64. Di Mascio P, Kaiser S, Sies H (1989) Lycopene as the most efficient biological carotenoid single oxygen quencher. Arch Biochem Biophys 274: 532-538.
65. Wang W, Vincour B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 218: 1-4.
66. Løvaas E (1991) Anti-oxidative effects of polyamines. J Am Oil Chem Soc 68: 353-358.
67. Verma S, Mishra SN (2005) Putrescine alleviation of growth in salt stressed Brassica juncea by inducing anti-oxidative defense system. J Plant Physiol 162: 669-677.
68. Saha J, Brauer EK, Sengupta A, Popescu SC, Gupta K, et al. (2015) Polyamines as redox homeostasis regulators during salt stress in plants. Front Environ Sci 3: 21.
69. Harborne JB (1999) Classes and functions of secondary products from plants. In: Chemicals from Plants, Perspectives on Secondary Plant Products; Walton NJ, Brown DE, Eds.; Imperial College Press: London, UK, pp: 1-25.
70. Bourgaud F, Gravot A, Milesi S, Gontier E (2001) Production of plant secondary metabolites: A historical perspective. Plant Sci 161: 839-851.
71. Verma N, Shukla S (2015) Impact of various factors responsible for fluctuation in plant secondary metabolites. J Appl Res Med Aromat Plants 2: 105-113.
72. Ferrandino A, Lovisolo C (2014) Abiotic stress effects on grapevine (Vitis vinifera L.): Focus on abscisic acid-mediated consequences on secondary metabolism and berry quality. Environ Exp Bot 103: 138-147.
73. Griesser M, Weingart G, Schoedl-Hummel K, Neumann N, Becker M, et al. (2015) Severe drought stress is affecting selected primary metabolites, polyphenols and volatile metabolites in grapevine leaves (Vitis vinifera cv. Pinot noir). Plant Physiol Biochem 88: 17-26.
74. Metlen KL, Aschehoug ET, Callaway RM (2015) Plant behavioral ecology: Dynamic plasticity in secondary metabolites. Plant Cell Environ 32: 641-653.
75. Musilova L, Ridl J, Polivkova M, Macek T, Uhlik O (2016) Effects of secondary plant metabolites on microbial populations: Changes in community structure and metabolic activity in contaminated environments. Int J Mol Sci 17: 1205.
76. Bennett RN, Wallsgrove RM (1994) Secondary metabolites in plant defense mechanisms. New Phytol 127: 617-633.
77. Radhakrishnan R, Lee IJ (2013) Spermine promotes acclimation to osmotic stress by modifying antioxidant, abscisic acid and jasmonic acid signals in soybean. J Plant Growth Regul 32: 22-30.
78. Asthir B, Koundal A, Bains NS (2012) Putrescine modulates antioxidant defense response in wheat under high temperature stress. Biologia Plantarum 56: 757-761.
79. Tanou G, Ziogas V, Belghazi M, Christou A, Filippou P, et al. (2014) Polyamines reprogram oxidative and nitrosative status and the proteome of citrus plants exposed to salinity stress. Plant Cell Environ 37: 864-885.
80. Zhang Y, Zhang H, Zou ZR, Liu Y, Hu XH (2015b) Deciphering the protective role of spermidine against saline-alkaline stress at physiological and proteomic levels in tomato. Phytochemistry 110: 13-21.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Proteomics and Bioinformatics (ISSN:2641-7561)